Human Oncology
Oncology
QBiotics' anticancer drug candidate tigilanol tiglate is an oncolytic small molecule, delivered intratumourally (usually single injection), that has potential in the treatment of a range of solid tumours.
While very effective as a monotherapy, murine data also supports the potential of tigilanol tiglate in combination with checkpoint inhibitors, conventional chemotherapy, and radiotherapy. Tigilanol tiglate is currently undergoing Phase II trials in patients with soft tissue sarcomas and head and neck cancers.
Tigilanol tiglate has been awarded Orphan Drug Designation by the FDA for the treatment of soft tissue sarcomas.

Tigilanol tiglate
Tigilanol tiglate has a multifactorial mode of action that leads to rapid destruction of injected tumours through tumour vasculature disruption and tumour cell oncolysis.
Tigilanol tiglate also induces systemic T cell responses via immunogenic cell death (ICD) induced by a caspase/gasdermin E-dependent pyroptopic pathway. Protein kinase C/C1 domain signaling (predominantly PKC β isoforms) appears necessary for tumour ablation in vivo and is also partly responsible for a strong wound healing response at the tumour site, with minimal scarring.
Strong efficacy signals from a first-in-human, dose-escalation safety trial
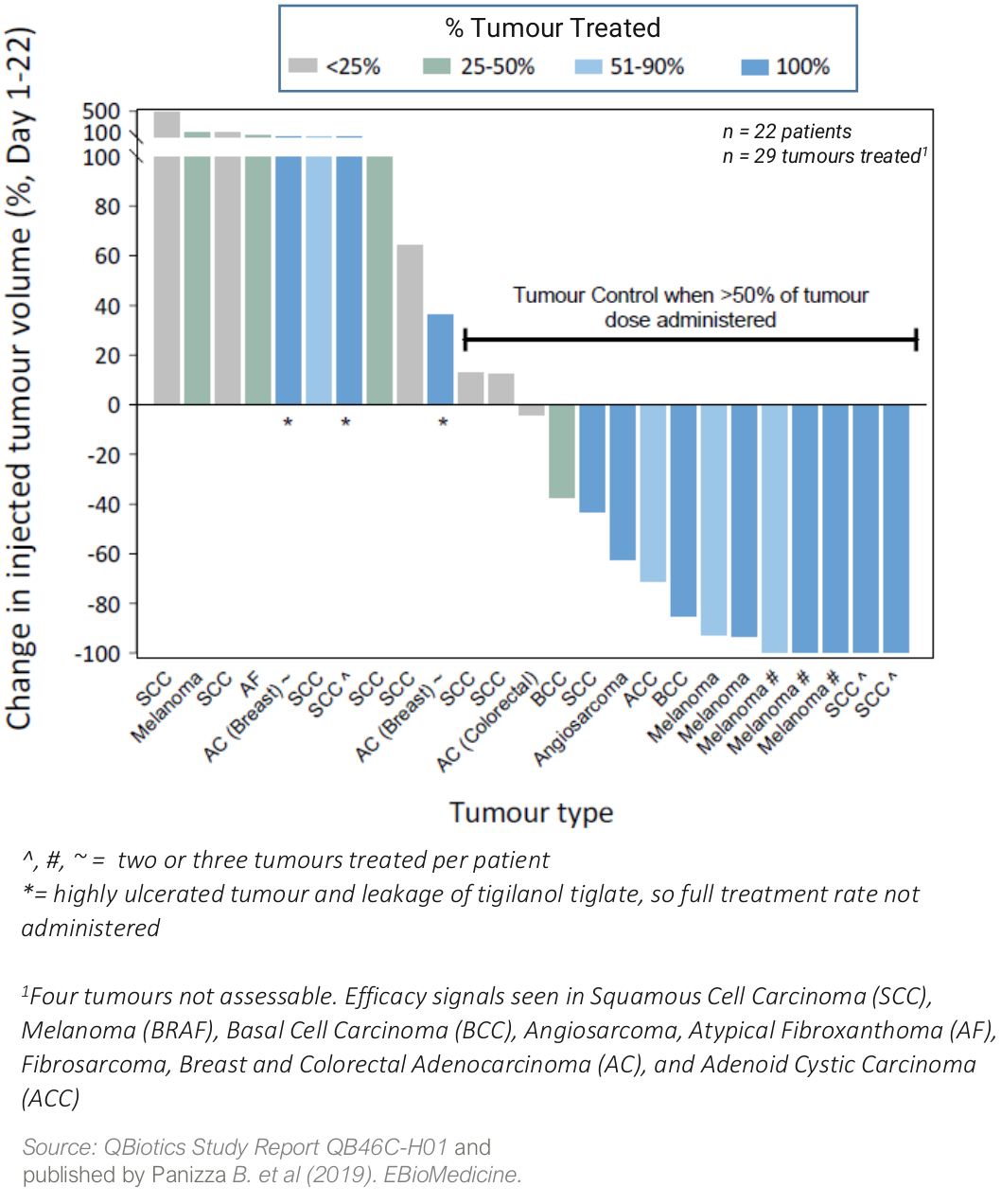
In a Phase I safety trial (QB46C-H01) in 22 patients with a broad range of cutaneous and subcutaneous solid tumors, tigilanol tiglate was well tolerated with a maximum tolerated dose (MTD) not reached, and signs of efficacy in nine different tumor types.
Four patients (18%) achieved a complete response in the injected tumour, two patients had partial responses (9%), while a further ten patients (45%) had stable disease. Two patients with metastatic melanoma also had non-injected tumor (abscopal) responses to tigilanol tiglate treatment.
Waterfall plot showing percentage change in tumour volume from Day 1 to Day 22 following a single injection with tigilanol tiglate (QB46-H01)
Immune response demonstrated
In a Phase I/II window of opportunity before surgery trial (QB46C-H03) in patients with head and neck squamous cell carcinoma (HNSCC), tigilanol tiglate induced immunogenic cell death (ICD) and CD8+ T cell infiltration in treated tumors.
Impressive canine veterinary data informs human development
The strong tumour responses observed in the human trials is supported by impressive efficacy data seen in dogs. Tigilanol tiglate is approved in the USA, EU, UK and AU, marketed as STELFONTA® *, as a first-line, alternative to surgery, veterinary pharmaceutical treatment for canine mast cell tumours (MCT).
*This link takes you to a site that may not comply with Australian regulations.
In a pivotal USA multi-centre trial in 123 canine patients with MCT, a single treatment induced a 75% complete response at 28 days post treatment, with an Objective Tumour Response of 80%1. Two injections led to an 88% complete response. There was no tumour recurrence in 89% of evaluable cases 12 months post-treatment.2
- De Ridder TR, et al. Journal Veterinary Internal Medincine 2020 ;doi :10.1111/jvim.15806.
- Jones, PD, et al. Recurrence‐free interval 12 months after local treatment of mast cell tumors in dogs using intratumoral injection of tigilanol tiglate. Journal of Veterinary Internal Medicine, 35(1), 451-455.
Wound Healing
QBiotics’ wound healing drug candidate, EBC-1013, is a topically applied, semi-synthetic small molecule, that has the potential for the treatment of a wide range of chronic and acute wounds and burns.
