QBiotics data presented at Society of Surgical Oncology Advanced Cancer Therapies 2025 Annual Meeting
BRISBANE, 17 February 2025
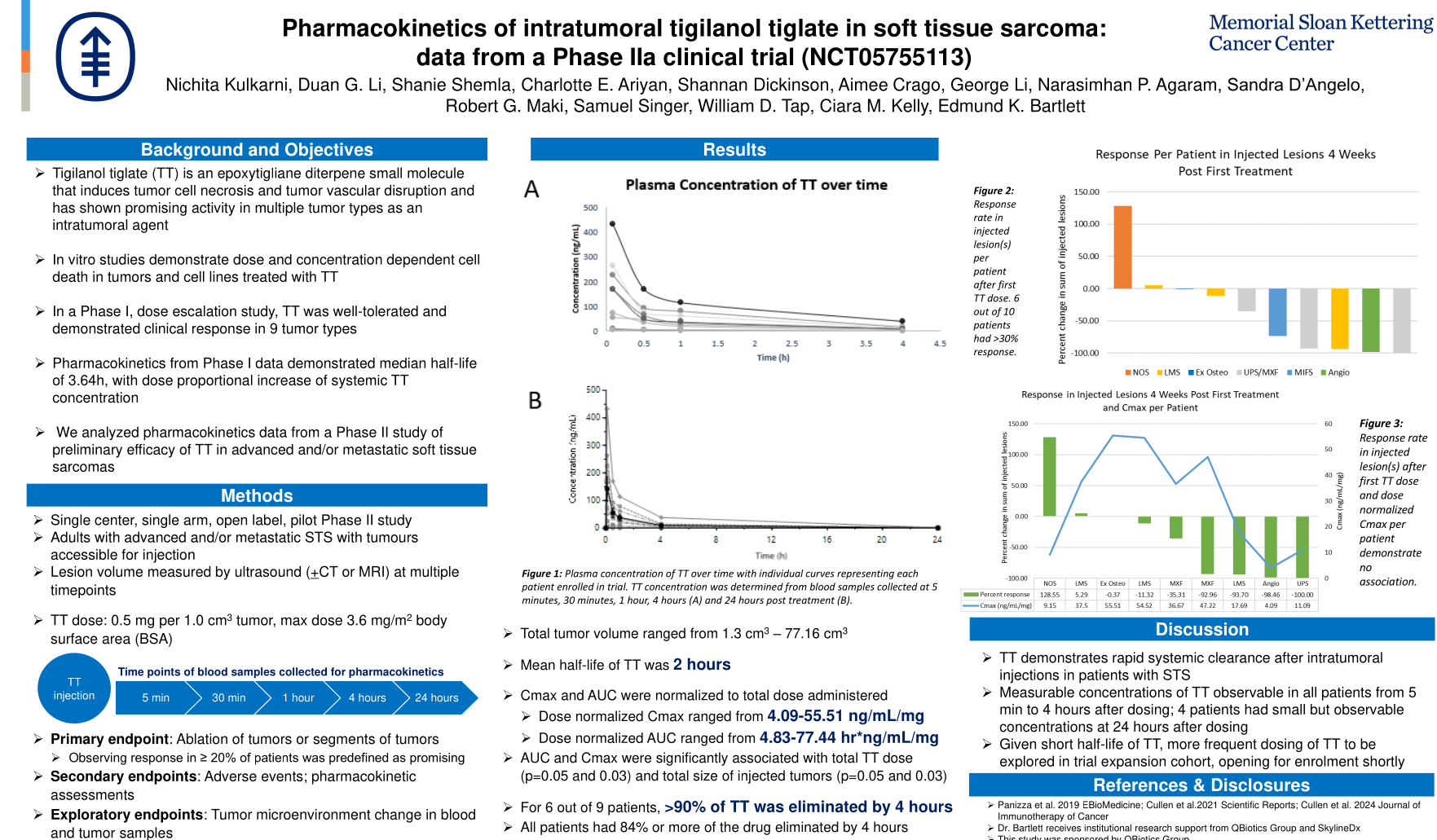
- Pharmacokinetic data from Phase IIa clinical trial (QB46C-H07) evaluating QBiotics’ small molecule, tigilanol tiglate in patients with advanced Soft Tissue Sarcoma (STS) presented at the Society of Surgical Oncology’s Advanced Cancer Therapies 2025 (ACT 2025) Annual Meeting (Scottsdale, AZ, US: 14-17 February 2025).
- This new data follows presentation of data from the Phase IIa trial at ESMO 2024 (Spain, September 2024) and CTOS 2024 (USA, November 2024) which highlighted that intratumoural treatment with tigilanol tiglate appeared safe for patients with STS, with local efficacy demonstrated and that patients with metastatic disease who were originally non-responsive to systemic therapies had responded to these therapies after treatment with tigilanol tiglate.
- ACT 2025 data demonstrates that tigilanol tiglate had rapid systemic clearance after intratumoural injections in patients with STS, with a mean half life of 2 hours and either nil or only small amounts measurable at 24 hours; given the short half life of tigilanol tiglate, more frequent dosing will be explored in the upcoming STS trial expansion.
QBiotics Group Limited (QBiotics) is pleased to share a poster presentation providing data on new pharmacokinetic findings from its Phase IIa clinical trial in Soft Tissue Sarcoma. These pharmacokinetic findings are in line with data from a previous Phase I study in skin and subcutaneous cancers.
The poster was presented at the Society of Surgical Oncology’s Advanced Cancer Therapies 2025 (ACT 2025) Annual Meeting (Scottsdale, AZ, US: 14-17 February 2025) by Nichita Kulkarni, MD from Memorial Sloan Kettering Cancer Center (New York, USA).
The Phase IIa trial completed recruitment in June 2024, following tigilanol tiglate being awarded Orphan Drug Designation for the treatment of STS by the United States Food and Drug Administration (FDA) in February 2024.
In September 2024, data was presented at the European Society for Medical Oncology (ESMO) 2024 conference, providing data demonstrating local efficacy of intratumoural treatment with tigilanol tiglate, and that treatment appears safe for patients with STS. Clinicians from the trial believed that the tolerability and activity observed warranted further investigation of tigilanol tiglate in patients with STS. This was followed by a presentation in November 2024 at the Connective Tissue Oncology Society (CTOS) 2024 Annual Meeting, which included additional observations made by the investigators outside of the clinical study, showing that patients with metastatic disease who were originally non-responsive to systemic therapy, responded to systemic therapy after tigilanol tiglate treatmented their primary tumour. These results encouraged QBiotics to expand the Phase IIa STS trial to investigate this response further, with an extension trial planned to initiate in Q1 2025.
At ACT 2025, the pharmacokinetic data collected through the Phase IIa was presented, with the key highlights being:
- Tigilanol tiglate demonstrates rapid systemic clearance after intratumoral injections in patients with STS;
- Measurable concentrations of tigilanol tiglate observable in all patients from 5 min to 4 hours after dosing; 4 patients had small but observable concentrations at 24 hours after dosing, and
- Given its short half-life, more frequent dosing of tigilanol tiglate is to be explored in the trial expansion cohort, which is opening for enrolment shortly.
QBiotics’ CEO, Stephen Doyle commented, “Investigating pharmacokinetics is essential for optimising the use of tigilanol tiglate in treating patients. We look forward to exploring the potential benefits of more frequent dosing in the upcoming trial expansion, which we expect to commence in the current quarter.
Our thanks to Dr. Nichita Kulkarni for presenting these data at the ACT 2025 Meeting, and to Dr. Edmund Bartlett and his entire team at Memorial Sloan Kettering Cancer Center for their excellent ongoing work on this trial.”
There were approximately 128,000 new cases of STS globally in 2023, with the incidence growing at 0.54% per year2.
ABOUT TRIAL QB46C-H07
QB46C-H07 (Clinical trial registration number NCT05755113) is a pilot Phase IIa, open label clinical trial to evaluate the preliminary efficacy and safety of intratumoural tigilanol tiglate in patients with a range of advanced and/or metastatic STS.
The Primary Endpoint is ablation rate defined as the proportion of patients achieving ≥30% reduction in tumour volume assessed by ultrasound compared to baseline.
The Secondary Endpoint is to assess safety and tolerability of tigilanol tiglate by assessing the incidence of adverse events and serious adverse events, and pharmacokinetics.
Exploratory Endpoints include local rate of recurrence at the injection site at 6 months post initial injection, and assessment of tumour response in biopsy samples. Biopsies were taken at baseline and at 14 days post-injection, and surgical specimens and blood samples will be used to assess changes in tumour biomarkers.
- Study authors: Edmund K. Bartlett, Duan G. Li, Shanie Shemla, Charlotte E. Ariyan, Shannan Dickinson, Aimee Crago, George Li, Narasimhan P. Agaram, Sandra D’Angelo, Robert G. Maki, Samuel Singer, William D. Tap, Ciara M. Kelly
- GlobalData®, American Cancer Society, Cancer Australia, Cancer Research UK, Canadian Cancer Society.
—ends—
FURTHER INFORMATION
STEPHEN DOYLE, CEO & MANAGING DIRECTOR
communications@qbiotics.com
or
MEDIA ENQUIRIES
JANE LOWE, IR DEPARTMENT
jane.lowe@irdepartment.com.au or +61 411 117 774
ABOUT QBIOTICS
QBiotics is an unlisted public Australian life sciences company that specialises in the discovery and development of novel cell signalling small molecules. QBiotics applies phenotypic screening to generate breakthrough innovation in the discovery of first in class solutions to challenging medical conditions. Our current clinical focus is on novel treatments for cancer and debilitating chronic wounds.
QBiotics’ lead oncology drug, tigilanol tiglate is currently in human clinical Phase II trials in Soft Tissue Sarcoma and Head and Neck Cancer. Tigilanol tiglate has received Orphan Drug Designation from the US FDA for the treatment of Soft Tissue Sarcomas. A veterinary formulation of tigilanol tiglate is registered and marketed as an oncology pharmaceutical, under the trade name STELFONTA®, in the USA, Europe, the UK and Australia.
QBiotics’ lead wound healing drug candidate, EBC 1013, is a small molecule targeting a range of wounds including chronic and acute wounds and burns. A first-in-human Phase I clinical development in patients with venous leg ulcers is open for recruitment.